In the second of our series of science commentaries Dr Ruth Peters discusses the methodology, directions and findings used in two recent reviews on dementia prevention.
Two recent reviews have approached the question of dementia prevention using very different methodologies and from different directions.
The Lancet Commission, ‘dementia prevention, intervention and care’ (1) and the National Academies of Science (NAS) report ‘preventing cognitive decline and dementia’ (2) based on the Agency for Healthcare Research and Quality (AHRQ) ‘Interventions to prevent age-related cognitive decline, mild cognitive impairment and clinical Alzheimer’s type dementia’ (3), were both presented at the Alzheimer’s Association International Conference (AAIC) in July 2017.
Both broadly aimed to consolidate the knowledge base about what can be done to prevent dementia. Both highlight increasing complexity in the field with one pointing to a lack of sufficiently robust evidence to underpin intervention messaging (2).
Different methodology
The methods used by the two reports differed.
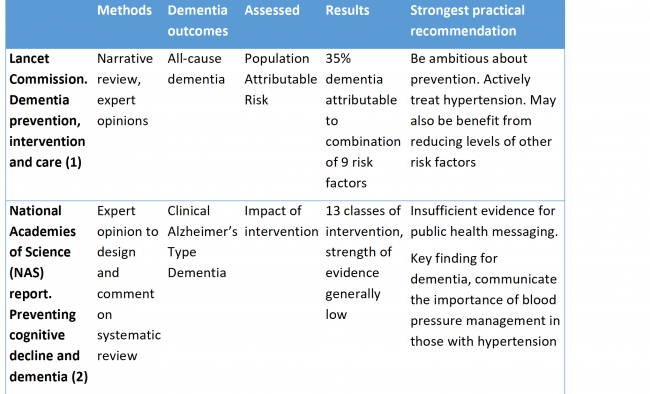
- Both examined the literature but the Lancet Commission used expert consensus and narrative. The NAS report used expert consensus to design a 2017 systematic review carried out by the Agency for Healthcare Research and Quality (AHRQ) and then commented on the results of the review (2,3).
- The Lancet Commission selected risk factors based on those detailed in the UK and USA guidelines and the NAS report on those reported in the AHRQ review. The AHRQ review in turn selected known risk factors and included only intervention studies with a minimum follow up between intervention and outcome of six months, among other inclusion criteria (3).
- The Lancet Review reported on all-cause dementia except for smoking where they reported on Alzheimer’s Disease whereas the NAS/AHRQ review reported on a selection of cognitive outcomes: delay or slowing of Age Related Cognitive Decline (ARCD); prevention, delay or slowing of Mild Cognitive Impairment (MCI); and Clinical Alzheimer’s Type Dementia (CATD). They did not report on all-cause dementia.
Results handled differently
The Lancet Commission focused on Population Attributable Risk (PAR) and used largely published data, Relative Risks (RR) and prevalence data. Where no pre-existing meta-analyses were available expert opinion was used to identify relevant publications and a novel meta-analysis was generated. Commonality of risk factors was taken into account.
The AHRQ review identified 263 eligible intervention studies using systematic review methodology but the variation in results was too great to allow meta-analysis. The AHRQ report also made a formal assessment of bias.
Findings also differ


Overall key practical recommendations are congruent, with both reports selecting active treatment of high blood pressure to potentially reduce risk.
Potential for bias
Both reports have strengths and flaws, in particular,
- The inclusion of hearing loss in the Lancet Commission report is a relatively novel and interesting addition but now also requires more robust evaluation with a full systematic review.
- The NAS focus on intervention is valuable since this will be key if we are to intervene. However, as the authors point out, intervention studies in this area are fraught with methodological issues and even the most robust double blind randomised placebo controlled trials are inevitably limited in their duration and probably too limited to really robustly evaluate impact of treatment on cognitive function.
The reliance on expert opinion is a strength, but this alongside inclusion of ‘known risk factors’ and selected portions of the evidence base runs the risk of reconfirming existing knowledge and omission of newly recognized or emergent risk factors and key subgroups or populations in whom prevention associations and messages may differ.
Over the last ten years the evidence base relating to dementia, risk factors and interventions has grown exponentially. It is probably no longer feasible for an in-depth systematic review of the entire area. Going forwards we will be increasingly basing our understanding on multiple reviews such as these, each potentially taking different perspectives and approaches (4). Critical appraisal will be key.
A very brief summary of the two reports
References
- Livingston G, Sommerlad A, Orgeta V, Costafreda S, Huntley J, Ames D, Ballard C, Banerjee S, Burns A, Cohen-Mansfield J, Cooper C, Fox N, Gitlin L, Howard R, Kales H, Larson E, Ritchie K, Rockwood K, Sampson E, Samus Q, Schneider L, Selbæk G, Teri L, Mukadam N. Dementia prevention, intervention, and care. The Lancet. July 19, 2017
- National Academies of Sciences, Engineering, and Medicine. 2017.
Preventing Cognitive Decline and Dementia: A Way Forward. Washington, DC: The National Academies Press. doi: https://doi.org/10.17226/24782.
- Kane RL, Butler M, Fink HA, Brasure M, Davila H, Desai P, Jutkowitz E, McCreedy E, Nelson VA, McCarten JR, Calvert C, Ratner E, Hemmy LS, Barclay T. Interventions To Prevent Age-Related Cognitive Decline, Mild Cognitive Impairment, and Clinical Alzheimer’s-Type Dementia. Comparative Effectiveness Review No. 188. (Prepared by the Minnesota Evidence-based Practice Center under Contract No. 290-2015-00008-I.) AHRQ Publication No. 17-EHC008-EF. Rockville, MD: Agency for Healthcare Research and Quality; March 2017. www.effectivehealthcare.ahrq.gov/reports/final.cfm.
doi: https://doi.org/10.23970/AHRQEPCCER188.
- Yaffe K. Modifiable risk factors and prevention of dementia. What is the latest evidence. JAMA Internal Medicine. 2018; 178:281-282
Terms and conditions
The recommendations, views or findings expressed in studies, trials or articles are expressly those of the authors. Such recommendations, views or findings do not represent the recommendations, views or findings of the International Research Network on Dementia Prevention (IRNDP), its Leadership Committee or Independent Advisory Group or any collaborating institutions. IRNDP accepts no responsibility for the accuracy or content, or liability in respect of studies and articles submitted to it.
Two high profile, commissioned, dementia prevention reviews 2017
Published by Heather Hubble on
Two recent reviews have approached the question of dementia prevention using very different methodologies and from different directions.
The Lancet Commission, ‘dementia prevention, intervention and care’ (1) and the National Academies of Science (NAS) report ‘preventing cognitive decline and dementia’ (2) based on the Agency for Healthcare Research and Quality (AHRQ) ‘Interventions to prevent age-related cognitive decline, mild cognitive impairment and clinical Alzheimer’s type dementia’ (3), were both presented at the Alzheimer’s Association International Conference (AAIC) in July 2017.
Both broadly aimed to consolidate the knowledge base about what can be done to prevent dementia. Both highlight increasing complexity in the field with one pointing to a lack of sufficiently robust evidence to underpin intervention messaging (2).
Different methodology
The methods used by the two reports differed.
Results handled differently
The Lancet Commission focused on Population Attributable Risk (PAR) and used largely published data, Relative Risks (RR) and prevalence data. Where no pre-existing meta-analyses were available expert opinion was used to identify relevant publications and a novel meta-analysis was generated. Commonality of risk factors was taken into account.
The AHRQ review identified 263 eligible intervention studies using systematic review methodology but the variation in results was too great to allow meta-analysis. The AHRQ report also made a formal assessment of bias.
Findings also differ
Overall key practical recommendations are congruent, with both reports selecting active treatment of high blood pressure to potentially reduce risk.
Potential for bias
Both reports have strengths and flaws, in particular,
The reliance on expert opinion is a strength, but this alongside inclusion of ‘known risk factors’ and selected portions of the evidence base runs the risk of reconfirming existing knowledge and omission of newly recognized or emergent risk factors and key subgroups or populations in whom prevention associations and messages may differ.
Over the last ten years the evidence base relating to dementia, risk factors and interventions has grown exponentially. It is probably no longer feasible for an in-depth systematic review of the entire area. Going forwards we will be increasingly basing our understanding on multiple reviews such as these, each potentially taking different perspectives and approaches (4). Critical appraisal will be key.
A very brief summary of the two reports
References
Preventing Cognitive Decline and Dementia: A Way Forward. Washington, DC: The National Academies Press. doi: https://doi.org/10.17226/24782.
doi: https://doi.org/10.23970/AHRQEPCCER188.
Terms and conditions
The recommendations, views or findings expressed in studies, trials or articles are expressly those of the authors. Such recommendations, views or findings do not represent the recommendations, views or findings of the International Research Network on Dementia Prevention (IRNDP), its Leadership Committee or Independent Advisory Group or any collaborating institutions. IRNDP accepts no responsibility for the accuracy or content, or liability in respect of studies and articles submitted to it.
Related Posts
General
Results from the our survey on dementia prevention, knowledge and barriers to risk reduction in Australian Primary Care settings
Australian Primary Care professionals who completed an online survey showed existing knowledge of the risk factors for dementia and were able to identify some hurdles that may need to be overcome to more successfully implement Read more…
Early Career Researchers
Career development in dementia research: The IRNDP Early and Mid-Career committee issue a call to action
The IRNDP Early and Mid-Career committee came together in 2020 to discuss and compare career development opportunities in their respective countries and decided to write a call to action! Their recommendations are now published online Read more…
Events
IRNDP Conference 2019
IRNDP held its first conference on the 14th and 15th of October, 2019 in Sydney. A big thank you to everyone who attended! There was a fantastic range of speakers from the United States, Canada, Read more…